Lakhmir Singh Manjit Kaur Solutions Chemical Reactions and Equations Part 1
Question 1: Why is respiration considered an exothermic process ? Solution : Respiration is an exothermic process because energy is produced during this process.
Question 2: On what basis is a chemical equation balanced ? Solution : A balanced chemical equation has equal number of atoms of different elements in the reactants and products.
Question 3: What happens chemically when quicklime is added to water filled in a bucket ? Solution : When quicklime is added to water, it forms slaked lime along with evolution of heat. There will be a rise in temperature of the bucket.
Question 4: Why should magnesium ribbon be cleaned before burning in air ? Solution : Magnesium ribbon should be cleaned before burning in air to remove the protective layer of basic magnesium carbonate from its surface.
Question 5: State whether the following statement is true or false : A chemical equation can be balanced easily by altering the formula of a reactant or product. Solution : False.
Question 6: What is wrong with the following chemical equation ? Mg + O ——— > MgO Correct and balance it. Solution : Oxygen should be in molecular form, O2 2Mg + O2 —–> 2MgO
Question 7: What does the symbol (aq) represent in a chemical equation ? Solution : The symbol (aq) represents aqueous solution in a chemical equation.
Question 8: Why is photosynthesis considered an endothermic reaction ? Solution : Photosynthesis is an endothermic reaction because sunlight energy is absorbed by green plants during this process.
Question 9: How will you indicate the following effects in a chemical equation ? (a) A solution made in water (b) Exothermic reaction © Endothermic reaction Solution : (a) Aqueous solution is indicated by the symbol ‘aq’. An exothermic reaction is indicated by writing “+Heat” or “+Heat energy” or “+Energy” on the products side of an equation. An endothermic reaction is indicated by writing “+Heat” or “+Heat energy” or “+Energy” on the reactants side of an equation.
Question 10: Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. (b) Phosphorus burns in oxygen to give phosphorus pentoxide. © Carbon disulphide burns in air to give carbon dioxide and sulphur dioxide. (d) Aluminium metal replaces iron from ferric oxide, Fe2 O3, giving aluminium oxide and iron. (e) Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate. Solution : (a) 2H2 S + 3O2 —–> 2H2 O + 2SO2 (b) P4 + 5O2 —–> 2P2 O5 © CS2 + 3O2 —–> CO2 + 2SO2 (d) 2Al + Fe2 O3 —–> Al2 O3 + 2Fe (e) BaCl2 + ZnSO4 —–> ZnCl2 + BaSO4
Question 11: Write the balanced chemical equations for the following reactions : (a) Calcium hydroxide + Carbon dioxide ——– > Calcium carbonate + Water (b) Aluminium + Copper chloride ——– > Aluminium chloride + Copper Solution : (a) Ca(OH)2 + CO2 —–> CaCO3 + H2 O 2Al + 3CuCl2 —–> 2AlCl3 + 3Cu
Lakhmir Singh Chemistry Class 10 Solutions Page No:19
Question 12: Complete and balance the following equations : (a) NaOH + ………… —–> Na2 S04 + H2 0 (b) Ca(OH)2 + ……….—–> CaC03 + H2 0 Solution : (a) 2NaOH + H2 SO4 —–> Na2 SO4 + 2H2 O (b) Ca(OH)2 + CO2 —–> CaCO3 + H2 O
Question 13: Correct and balance the following equations :
- Ca + H2 0 — > CaOH + H
- N + H — > NH3
Solution :
- Ca + 2H2 O —–> Ca(OH)2 + H2
- N2 + 3H2 —–> 2NH3
Question 14: Write complete balanced equations for the following reactions : (a) Calcium (solid) + Water (liquid) —–> Calcium hydroxide (solution) + Hydrogen (gas) (b) Sulphur dioxide (gas) + Oxygen (gas) —–> Sulphur trioxide (gas) Solution : (a) Ca (s) + 2H2 O(l) —–> Ca(OH)2 (aq) + H2 (g) (b) 2SO2 (g) + O2 (g) —–> 2SO3 (g)
Question 15:
- Na + O2 —–> Na2 O
- H2 O2 —–> H2 O + O2
- Mg(OH)2 + HCl —–> MgCl2 + H2 O.
- Fe + O2 —–> Fe2 O3
- Al(OH)3 —–> Al2 O3 + H2 O
- NH3 + CuO —–> Cu + N2 + H2 O
- Al2 (SO4)3 + NaOH —–> Al(OH)3 + Na2 SO4
- HNO3 + Ca(OH)2 —–> Ca(NO3)2 + H2 O
- NaOH + H2 SO4 —–> Na2 SO4 + H2 O
- BaCl2 + H2 SO4 —–> BaSO4 + HCl
Solution :
- 4Na + O2 —–> 2Na2 O
- 2H2 O2 —–> 2H2 O + O2
- Mg(OH)2 + 2HCl —–> MgCl2 + 2H2 O.
- 4Fe + 3O2 —–> 2Fe2 O3
- 2Al(OH)3 —–> Al2 O3 + 3H2 O
- 2NH3 + 3CuO —–> 3Cu + N2 + 3H2 O
- Al2 (SO4)3 + 6NaOH —–> 2Al(OH)3 + 3Na2 SO4
- 2HNO3 + Ca(OH)2 —–> Ca(NO3)2 + 2H2 O
- 2NaOH + H2 SO4 —–> Na2 SO4 + 2H2 O
- BaCl2 + H2 SO4 —–> BaSO4 + 2HCl
Question 16: Fill in the following blanks with suitable words : (a) Chemical equations are balanced to satisfy the law of……. (b) A solution made in water is known as an………. solution and indicated by the symbol……………. Solution : (a) Conservation of mass (b) Aqueous; (aq)
Question 17: (a) Give one example of a chemical reaction. (b) State two characteristics of the chemical reaction which takes place when dilute sulphuric acid is poured over zinc granules. © Give two characteristics of the chemical reaction which occurs on adding potassium iodide solution to lead nitrate solution. Solution : (a) Magnesium Ribbon is heated in the presence of air to form a white powder called magnesium oxide. (b) When dilute sulphuric acid is poured over zinc granules
- there will be a rise in temperature
- evolution of hydrogen gas.
©
- A yellow precipitate is formed.
- There will be a change in color (from colourless to yellow).
Question 18: (a) What is a chemical equation ? Explain with the help of an example. (b) Giving examples, state the difference between balanced and unbalanced chemical equations. © Balance the following chemical equations :
- NH3 —–> N2 + H2
- C +C02 —–> CO
Solution : (a) The method of representing a chemical reaction with the help of symbols and formulae of substances involved in it is called a chemical equation. Example: Zinc metal reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas. This equation is written as: Zn + H2 SO4 —–> ZnSO4 + H2 (b) A balanced chemical equation has an equal number of atoms of different elements in the reactants and products. It has equal masses of various elements in the reactants and products. Example: Zn + H2 SO4 —–> ZnSO4 + H2 An unbalanced chemical equation has an unequal number of atoms of one or more elements in the reactants and products. It has unequal masses of various elements in the reactants and products. Example: H2 + O2 —–> 2H2 O ©
- 2NH3 —–> N2 + 3H2
- C + CO2 —–> 2CO
Question 19: When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are :
- elements
- compounds
- reactants
- products
- metals
- non-metals
Solution : H2 + CuO —–> Cu + H2 O
- Elements : H2 and Cu
- Compounds : CuO and H2 O
- Reactants: H2 and CuO
- Products: Cu
- Metal: Cu
- Non-metal: H2
Question 20: (a) What are the various ways in which a chemical equation can be made more informative ? Give examples to illustrate your answer. (b) Write balanced chemical equation from the following information : An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water. Solution : (a) The various ways in which a chemical equation can be made more informative are : (i) By indicating the physical states of the reactants and products. Example: Gaseous state is indicated by the symbol (g). Zn (s) + H2 SO4 (aq) —–> ZnSO4 (aq) + H2 (g) (ii) By indicating the heat changes taking place in the reaction. For xxample: An exothermic reaction is indicated by writing “+Heat” or “+Heat energy” or “+Energy” on the products side of an equation. C (s) + O2 (g) —–> CO2 (g) + Heat (iii) By indicating the “conditions” under which the reaction takes place. Example: Delta stands for heat which is written over the arrow of the equation.
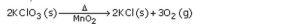
(b) Ca(OH)2 (aq) + CO2 (g) —–> CaCO3 (s) + H2 O (l)
Question 21: (a) What is a balanced chemical equation ? Why should chemical equations be balanced ? (b) Aluminium burns in chlorine to form aluminium chloride (AlCl3). Write a balanced chemical equation for this reaction. © Potassium metal reacts with water to give potassium hydroxide and hydrogen gas. Write a balanced chemical equation for this reaction. Solution : (a) A balanced chemical equation has an equal number of atoms of different elements in the reactants and products. It has equal masses of various elements in the reactants and products. A chemical equation should be balanced to satisfy the law of conservation of chemical reactions. (b) 2Al + 3Cl2 —–> 2AlCl3 © 2K + 2H2 O —–> 2KOH + H2
Lakhmir Singh Chemistry Class 10 Solutions Page No:20
Question 22: (a) Explain, with example, how the physical states of the reactants and products can be shown in a chemical equation. (b) Balance the following equation and add state symbols : Zn + HCl ——– > ZnCl2 + H2 © Convey the following information in the form of a balanced chemical equation : “An aqueous solution of ferrous sulphate reacts with an aqueous solution of sodium hydroxide to form a precipitate of ferrous hydroxide and sodium sulphate remains in solution.” Solution : (a) The physical states of the reactants and products are shown by putting the “state symbols” in an equation. For example: Zn (s) + H2 SO4 (aq) —–> ZnSO4 (aq) + H2 (g) b) Zn (s) + 2HCl(aq) —–> ZnCl2 (aq) + H2 (g) © FeSO4 (aq)+ 2NaOH (aq) —–> Fe(OH)2 (s) + Na2 SO4 (aq)
Question 23: Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer. Solution :
- Evolution of gas. For example: When sodium carbonate reacts with dilute hydrochloric acid, carbon dioxide gas is evolved.
- Formation of a precipitate. For example: When potassium iodide solution is added to a solution of lead nitrate, yellow precipitate of lead iodide is formed.
Question 24: (a) Aluminium hydroxide reacts with sulphuric acid to form aluminium sulphate and water. Write a balanced equation for this reaction. (b) Balance the following chemical equation : MnO2 + HCl —–> MnCl2 + Cl2 + H2 O Solution : (a) 2Al(OH)3 + 3H2 SO4 —–> Al2 (SO4)3 + 6H2 O (b) MnO2 + 4HCl —–> MnCl2 + Cl2 + 2H2 O
Question 25: Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide reacts with sulphuric acid to produce sodium sulphate and water. Solution : (a) MgCO3 (s) + 2HCl (aq) MgCl2 (aq) + CO2 (g) + H2 O (l) (b) 2NaOH (aq) + H2 SO4 (aq) Na2 SO4 (aq) + 2H2 O (l)
Question 26: Carbon monoxide reacts with hydrogen under certain conditions to form methanol (CH3 OH). Write a balanced chemical equation for this reaction indicating the physical states of reactants and product as well as the conditions under which this reaction takes place. Solution :

Question 27: (a) Potassium chlorate (KClO3) on heating forms potassium chloride and oxygen. Write a balanced equation for this reaction and indicate the evolution of gas. (b) Rewrite the following information in the form of a balanced chemical equation : Magnesium burns in carbon dioxide to form magnesium oxide and carbon. Solution : (a) 2KClO3 (s) 2KCl (s) + 3O2 (g) (b) 2Mg + CO2 —–> 2MgO + C
Question 28: (a) Substitute formulae for names and balance the following equation : Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas. (b) Write balanced chemical equation with state symbols for the following reaction : Sodium hydroxide solution reacts with hydrochloric acid solution to produce sodium chloride solution and water. Solution : (a) CaCO3 + 2HCl —–> CaCl2 + H2 O + CO2 (b) NaOH (aq) + HCl(aq) —–> NaCl (aq) + H2 O (l)
Question 29: Ammonia reacts with oxygen to form nitrogen and water. Write a balanced chemical equation for this reaction. Add the state symbols for all the reactants and products. Solution : 4NH3 (g)+ 3O2 (g) —–> 2N2 (g) + 6H2 O (l)
Question 30: Write a balanced chemical equation for the process of photosynthesis giving the physical states of all the substances involved and the conditions of the reaction. Solution : 6CO2 (g) + 6H2 O C6 —–> H12 O6 (aq) + 6O2 (g) Carbondioxide Water Glucose Oxygen
Question 31: Translate the following statement into chemical equation and then balance it : Barium chloride solution reacts with aluminium sulphate solution to form a precipitate of barium sulphate and aluminium chloride solution. Solution : 3BaCl2 (aq) + Al2 (SO4)3 (aq) —–> 3BaSO4 (s) + 2AlCl3 (aq)
Question 32: When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products. Solution : 2KNO3 (s) —–> 2KNO2 (s) + O2 (g)
Question 33: (a) What is meant by a chemical reaction ? Explain with the help of an example. (b) Give one example each of a chemical reaction characterised by :
- evolution of a gas
- change in colour
- formation of a precipitate
- change in temperature
- change in state.
Solution : (a) Chemical reactions are the processes in which new substances with new properties are formed. For example: When magnesium ribbon is heated, it burns in air to form a white powder called magnesium oxide. (b)
- The chemical reaction between zinc and dilute sulphuric acid.
- The chemical reaction between citric acid and purple coloured potassium permanganate solution is characterised by change in colour (from purple to colourless).
- The chemical reaction between potassium iodide and lead nitrate is characterised by the formation of a yellow precipitate of lead iodide.
- The reaction between quick lime and water to form slaked lime is characterised by a change in temperature.
- When wax is burned, then water and carbon dioxide are formed. Wax is a solid; water is a liquid whereas carbon dioxide is a gas.
Lakhmir Singh Chemistry Class 10 Solutions Page No:21
Question 34: (a) State the various characteristics of chemical reactions. (b) State one characteristic each of the chemical reaction which takes place when :
- dilute hydrochloric acid is added to sodium carbonate
- lemon juice is added gradually to potassium permanganate solution
- dilute sulphuric acid is added to barium chloride solution
- quicklime is treated with water
- wax is burned in the form of a candle
Solution : (a) The various characteristics of chemical reactions are:
- Evolution of a gas
- Formation of a precipitate
- Change in colour
- Change in temperature
- Change in state.
(b)
- Evolution of carbon dioxide gas
- Change in colour from purple to colourless
- Formation of white precipitate of barium sulphate
- Change in temperature
- Change in state from solid to liquid and gas.
Question 35: (a) What do you understand by exothermic and endothermic reactions ? (b) Give one example of an exothermic reaction and one of an endothermic reaction. © Which of the following are endothermic reactions and which are exothermic reactions ?
- Burning of natural gas
- Photosynthesis
- Electrolysis of water
- Respiration
- Decomposition of calcium carbonate
Solution : (a) Those reactions in which heat is evolved are known as exothermic reactions. The reactions in which heat is absorbed are known as endothermic reactions. (b) Example of exothermic reaction: C (s) + O2 (g) —–> CO2 + Heat Example of endothermic reaction: N2 (g) + O2 (g) + Heat —–> 2NO (g) © Endothermic reactions: Photosynthesis, Electrolysis of water, Decomposition of calcium carbonate. Exothermic reactions: Burning of natural gas, Respiration.
Lakhmir Singh Chemistry Class 10 Solutions Page No:22
Question 46: When the solution of substance X is added to a solution of potassium iodide, then a yellow solid separates out from the solution. (a) What do you think substance X is likely to be ? (b) Name the substance which the yellow solid consists of. © Which characteristic of chemical reactions is illustrated by this example ? (d) Write a balanced chemical equation for the reaction which takes place. Mention the physical states of all the reactants and products involved in the chemical equation. Solution : (a) Lead nitrate. (b) Lead iodide. © Formation of a precipitate. (d) Pb(NO3)2 (aq) + 2KI (aq) —–> PbI2 (s) + 2KNO3 (aq)
Question 47: When water is added gradually to a white solid X, a hissing sound is heard and a lot of heat is produced forming a product Y. A suspension of Y in water is applied to the walls of a house during white washing. A clear solution of Y is also used for testing carbon dioxide gas in the laboratory. (a) What could be solid X ? Write its chemical formula. (b) What could be product Y ? Write its chemical formula. © What is the common name of the solution of Y which is used for testing carbon dioxide gas ? (d) Write chemical equation of the reaction which takes place on adding water to solid X. (e) Which characteristic of chemical reactions is illustrated by this example ? Solution : (a) Calcium oxide, CaO. (b) Calcium hydroxide, Ca(OH)2 © Lime water. (d) CaO + H2 O —–> Ca(OH)2 (e) Change in temperature.
Question 48: When metal X is treated with a dilute acid Y, then a gas Z is evolved which burns readily by making a little explosion. (a) Name any two metals which can behave like metal X. (b) Name any two acids which can behave like acid Y. © Name the gas Z. (d) Is the gas Z lighter than or heavier than air ? (e)Is the reaction between metal X and dilute acid Y exothermic or endothermic ? (f) By taking a specific example of metal X and dilute acid Y, write a balanced chemical equation for the reaction which takes place. Also indicate physical states of all the reactants and products. Solution : (a) Zinc and Iron. (b) Dilute hydrochloric acid and dilute sulphuric acid. © Hydrogen. (d) Lighter than air. (e) Exothermic. (f) Suppose metal X is zinc (Zn) and acid Y is dilute hydrochloric acid (HCl) ; Zn (s) + 2HCl (aq) ZnCl2 (aq) + H2 (g)
Question 49: A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P. (a) What is substance P ? Write its common name as well as chemical formula. (b) What is substance Q ? © What is gas R ? (d) What is substance S ? What is its clear solution known as ? (e) What is substance T ? Name any two natural forms in which substance T occurs in nature. Solution : (a) Calcium carbonate (limestone), CaCO3 (b) Calcium oxide, CaO © Carbon dioxide, CO2 (d) Calcium hydroxide, Ca(OH)2; Lime water. (e) Calcium carbonate; Limestone and Marble.
Lakhmir Singh Chemistry Class 10 Solutions Page No:23
Question 50: A silvery-white metal X taken in the form of ribbon, when ignited, burns in air with a dazzling white flame to form a white powder Y. When water is added to powder Y, it dissolves partially to form another substance Z. (a) What could metal X be ? (b) What is powder Y ? © With which substance metal X combines to form powder Y ? (d) What is substance Z ? Name one domestic use of substance Z. (e) Write a balanced chemical equation of the reaction which takes place when metal X burns in air to form powder Y. Solution : (a) Magnesium, Mg. (b) Magnesium oxide,MgO © Oxygen (of air),O2 (d) Magnesiumhydroxide, Mg(OH)2; Used as antacid to relieve indigestion (e) 2Mg + O2 —–> 2MgO
Question 51: A metal X forms a salt XSO4. The salt XSO4 forms a clear solution in water which reacts with sodium hydroxide solution to form a blue precipitate Y. Metal X is used in making electric wires and alloys like brass. (a) What do you think metal X could be ? (b) Write the name, formula and colour of salt XSO4. © What is the blue precipitate Y ? (d) Write a chemical equation of the reaction which takes place when salt XSO4 reacts with sodium hydroxide solution. Give the state symbols of all the reactants and products which occur in the above equation. Solution : (a) Copper, Cu. (b) Copper sulphate, CuSO4, Blue colour. © Copper hydroxide, Cu(OH)2 (d) CuSO4 (aq) + 2NaOH (aq) —–> Cu(OH)2 (s) + Na2 S04 (aq)
Question 52: The metal M reacts vigorously with water to form a solution S and a gas G. The solution S turns red litmus to blue whereas gas G, which is lighter than air, burns with a pop sound. Metal M has a low melting point and it is used as a coolant in nuclear reactors. (a) What is metal M ? (b) What is solution S ? Is it acidic or alkaline ? © What is gas G ? (d) Write a balanced chemical equation for the reaction which takes place when metal M reacts with water. (e) Is this reaction exothermic or endothermic ? Solution : (a) Sodium, Na. (b) Sodium hydroxide solution (NaOH solution), Alkaline. © Hydrogen, H2 (d) 2Na + 2H2 O —–> 2NaOH + H2 (e) Exothermic.
Question 53: When a mixture of gases X and Y is compressed to 300 atm pressure and then passed over a catalyst consisting of a mixture of zinc oxide and chromium oxide (heated to a temperature of 300°C), then an organic compound Z having the molecular formula CH4 O is formed. X is a highly poisonous gas which is formed in appreciable amounts when a fuel burns in a limited supply of air ; Y is a gas which can be made by the action of a dilute acid on an active metal; and Z is a liquid organic compound which can react with sodium metal to produce hydrogen gas. (a) What are X, Y and Z ? (b) Write a balanced chemical equation of the reaction which takes place when X and Y combine to form Z. Indicate the conditions under which the reaction occurs. Solution : (a) X is carbon monoxide gas (CO); Y is hydrogen gas (H2) ; Z is methanol (or Methyl alcohol) (CH3 OH) (CH4 0 = CH3 OH) (b) Formation of Z:

Question 54: The white solid compound A decomposes quite rapidly on heating in the presence of a black substance X to form a solid compound B and a gas C. When an aqueous solution of compound B is reacted with silver nitrate solution, then a white precipitate of silver chloride is obtained along with potassium nitrate solution. Gas C does not burn itself but helps burn other things. (a) What is compound A ? (b) What is compound B ? © What is gas C ? (d) What do you think is the black substance X ? What is its function ? (e) What is the general name of substances like X ? Solution : (a) Potassium chlorate, KClO3 (b) Potassium chloride, KCl © Oxygen, O2 (d) Manganese dioxide, MnO2; It acts as a catalyst in the decomposition of potassium chlorate to form oxygen gas (e) Catalysts
Question 55: Gas A, which is the major cause of global warming, combines with hydrogen oxide B in nature in the presence of an environmental factor C and a green material D to form a six carbon organic compound E and a gas F. The gas F is necessary for breathing. (a) What is gas A ? (b) What is the common name of B ? © What do you think could be C ? (d) What is material D ? Where is it found ? Solution : (a) Carbon dioxide,CO2 (b) Water, H2 O © Sunlight. (d) Chlorophyll;Green leaves of plants. (e) Glucose, C6 H12 O6 (f) Oxygen; Photosynthesis.
Lakhmir Singh Chemistry Class 10 Solutions Page No:45
Question 1: What type of reaction is represented by the digestion of food in our body ? Solution : Decomposition reaction.
Question 2: Name the various types of chemical reactions. Solution : The various types of chemical reactions are:
- Combination reactions.
- Decomposition reaction.
- Displacement reaction.
- Double displacement reaction.
- Oxidation and reduction reactions.
Question 3: Why does-the colour of copper sulphate solution change when an iron nail is kept immersed in it ? Solution : The colour of copper sulphate solution changes when iron nail is kept immersed in it due to the displacement reaction taking place between iron and copper leading to formation of iron sulphate.
Question 4: Write the balanced chemical equation for the following reaction : Zinc + Silver nitrate —–> Zinc nitrate + Silver Solution : Zn + 2AgNO3 —–> Zn(NO3)2 + 2Ag
Question 5: Which term is used to indicate the development of unpleasant smell and taste in fat and oil containing foods due to aerial oxidation (when they are kept exposed for a considerable time) ? Solution : Rancidity.
Question 6: What is the general name of the chemicals which are added to fat and oil containing foods to prevent the development of rancidity Solution : Anti-oxidants.
Question 7: State an important use of decomposition reactions. Solution : The digestion of food in the body is an example of decomposition reaction.
Question 8: What are anti-oxidants ? Why are they added to fat and oil containing foods ? Solution : Anti-oxidant is a substance which prevents oxidation. They are added to fat and oil containing foods so that they do not get oxidized easily and hence do not turn rancid.
Question 9: Explain why, food products containing fats and oils (like potato chips) are packaged in nitrogen. Solution : Food products containing fats and oils are packaged in nitrogen so that there is no oxygen to cause oxidation of food and make it rancid.
Question 10: Give one example of a decomposition reaction which is carried out: (a) with electricity (b) by applying heat Solution : (a) When fused sodium chloride is decomposed by passing electricity, sodium metal is obtained. (b) When lead nitrate is heated strongly, it breaks down to form lead monoxide, nitrogen dioxide and oxygen.
Question 11: What type of chemical reaction is used to extract metals from their naturally occurring compounds like oxides or chlorides ? Solution : Decomposition reactions (carried out by electricity).
Question 12: Name two anti-oxidants which are usually added to fat and oil containing foods to prevent rancidity. Solution : BHA (Butylated Hydroxy Anisole) and BHT (Butylated Hydroxy Toluene).
Question 13: Write one equation each for the decomposition reactions where energy is supplied in the form of (a) heat, (b) light, and © electricity. Solution :
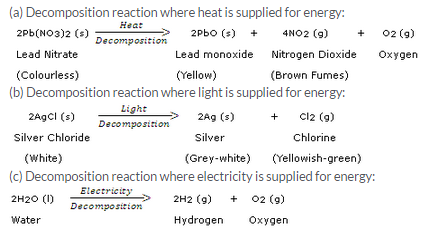
Question 14: In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the chemical equation of the reaction involved. Solution : 2AgNO3 (aq) + Cu (s) —–>Cu(NO3)2 (aq) + 2Ag (s)
Question 15: What type of reactions are represented by the following equations ?
- CaC03 ——- > CaO + C02
- CaO + H2 0 ——- > Ca(OH)2
- 2FeSO 4 ——- >Fe2 03 + S02 + S03
- NH4 Cl —–> NH3 +HCl
- 2Ca+O2 ——- > 2CaO
Solution :
- Decomposition.
- Combination.
- Decomposition.
- Decomposition.
- Combination.